The Lancet Neurology (influence factor IF=28.755) published online on April 22, 2020 The results of a large randomized controlled clinical trial led by Professor Shi Fudong of Neurology of Tianjin Medical University General Hospital and Beijing Tiantan Hospital and participated by six hospitals across China. This trial reported that Tocilizumab, an IL-6R block-blocking agent, had a significant advantage over Azathioprine, an immunosuppressive agent, in reducing the recurrence and disability rates in patients with Neuromyelitis Optica Spectrum disorder (NMOSD), providing advanced (level I) evidence-based medicine for the treatment of the disease. Link: Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open-label, multicentre, randomised, phase 2 trial
NMOSD is a highly recurrent, highly disabling autoimmune disease of the central nervous system, characterized by recurrent optic neuritis and myelitis. Since the disease was established as an independent disease in 2004, there are no guidelines for immunotherapy to prevent recurrence. At present, the treatment of NMOSD in China is similar to that in other countries in the world, mainly including classic immunosuppressive agents such as azathioprine, morticophenol and CD20 monoclonal antibody rituximab. Even with the use of these drugs, 25-50% of patients experienced a recurrence of the disease during follow-up. Each relapse leads to a buildup of disability, with the patient's nervous system unable to function at the same level as before the relapse. In 2019, the results of three foreign multicentre randomized controlled trials suggested that complement inhibitors Eculizumab, CD19 Inebilizumab, and IL-6R Satralizumab significantly reduced the risk of NMOSD recurrence compared with placebo. Eculizumab has been approved by the US Food and Drug Administration for the treatment of NMOSD. There are no clinical studies comparing the safety and efficacy of the new targeted drugs with the classic immunosuppressive drugs in long-term treatment. To solve the above problems, Professor Shi's team, based on the exploration of the pathogenesis of NMOSD, carried out the first clinical study of topizumab, another drug targeting IL-6R, and compared with azathioprine to treat NMOSD head to head (referred to as the TANGO trial). Tozumab is a monoclonal antibody against the humanized IL-6 receptor (IL-6R), which can inhibit the pro-IL-6 signaling pathway and prevent abnormal activation of peripheral B cells. The efficacy of tozumab in NMOSD patients needs to be confirmed urgently. Azathioprine is an immunosuppressive drug that inhibits purine nucleotide biosynthesis. Azathioprine is currently one of the first-line drugs for the treatment of NMOSD, but it has side effects such as leukocytosis and liver dysfunction.
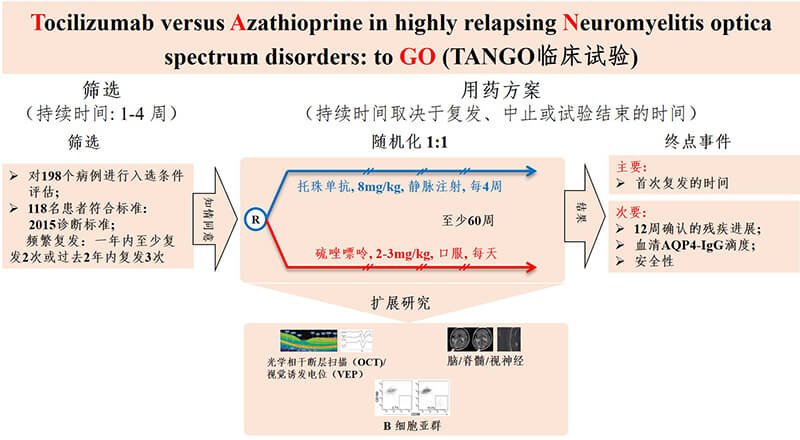
Figure 1. TANGO Trials. gov.number: NCT03350633
From November 2017 to August 2018, TANGO recruited 118 patients diagnosed with clinically high recurrence of NMOSD, about 40% of whom had other autoimmune diseases. These patients had at least two relapses in the year before randomization, or at least three recurrences in the year before randomization. All patients were randomly divided into the tozumab group and the azathioprine group in a 1:1 ratio, with 59 patients in each group. Patients in the tozumab group received 8mg/kg/m intravenous administration, and patients with azathioprine received 2-3mg/kg/d oral administration. After 12 - and 24-week washout periods, patients were treated with a monotherapy regimen. The primary endpoint was recurrence since drug initiation (Figure 1). Compared with azathioprine, tozumab significantly extended the time interval from randomization to disease recurrence (median 78.9 weeks in the tozumab group vs. median 56.7 weeks in the azathioprine group, P=0.0026). By the end of the trial, only eight patients in the tozumab group had relapsed, compared with 28 in the azathioprine group. Compared with azathioprine, tozumab significantly reduced the risk of recurrence by 76.4% (hazard ratio [HR]=0.236, 95% confidence interval [CI]: 0.107-0.518; P<0·0001) (Figure 2). At the monotherapy stage, tozumab reduced the risk of recurrence by 81.2% (HR=0.188, 95%CI: 0.076-0.463) in the tozumab group and azathioprine group, with 6 and 23 cases of recurrence, respectively. P < 0.0001). Compared with azathioprine, the incidence of 12-week disease and disability progression was significantly lower in the tozumab group (HR=0.288, 95%CI: 0.105-0.795; P=0.0087), the titer of peripheral blood aquaporin 4 autoantibody (AQP4-IGG) decreased by 50%, while the titer of serum AQP4-IGG in azathioprine group did not change significantly during the whole treatment period. Stratified analysis of patients with or without other autoimmune diseases showed that tozumab significantly reduced the risk of recurrence compared with azathioprine (Figure 3), regardless of whether the patients had other autoimmune diseases. From the perspective of safety, the incidence of side effects of tozumab and azathioprine is basically the same, and most of the adverse reactions are mild to moderate, such as upper respiratory tract infection and liver dysfunction. However, the incidence of treatment-related side effects was lower in the tozumab group (61%) than in the azathioprine group (83%).
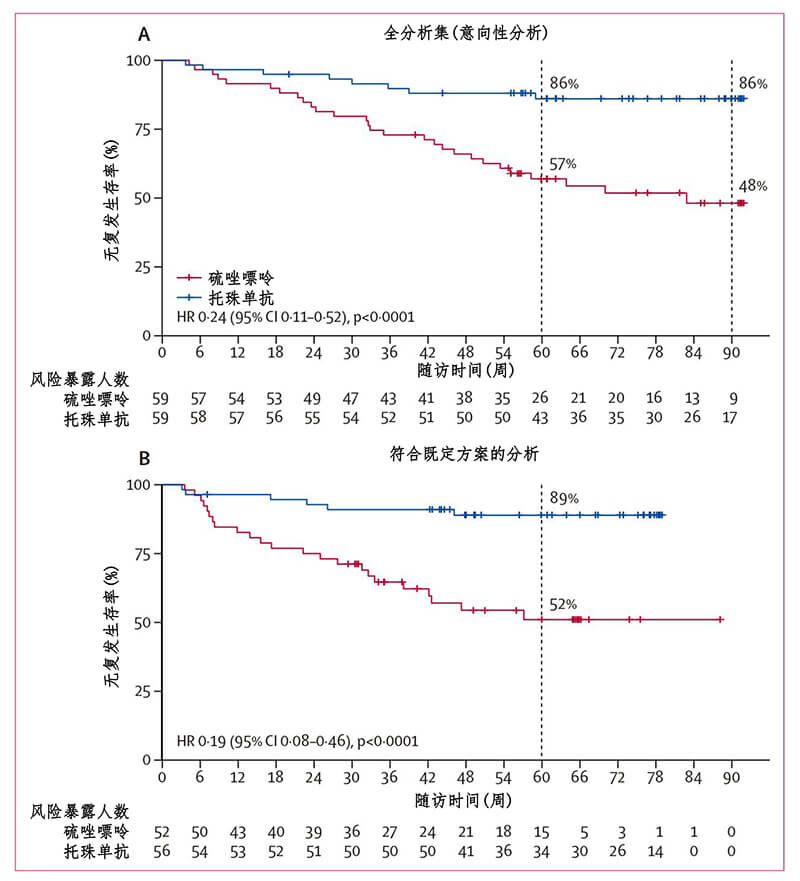
Figure 2. TANGO trial primary endpoint event (NMOSD recurrence since randomization)
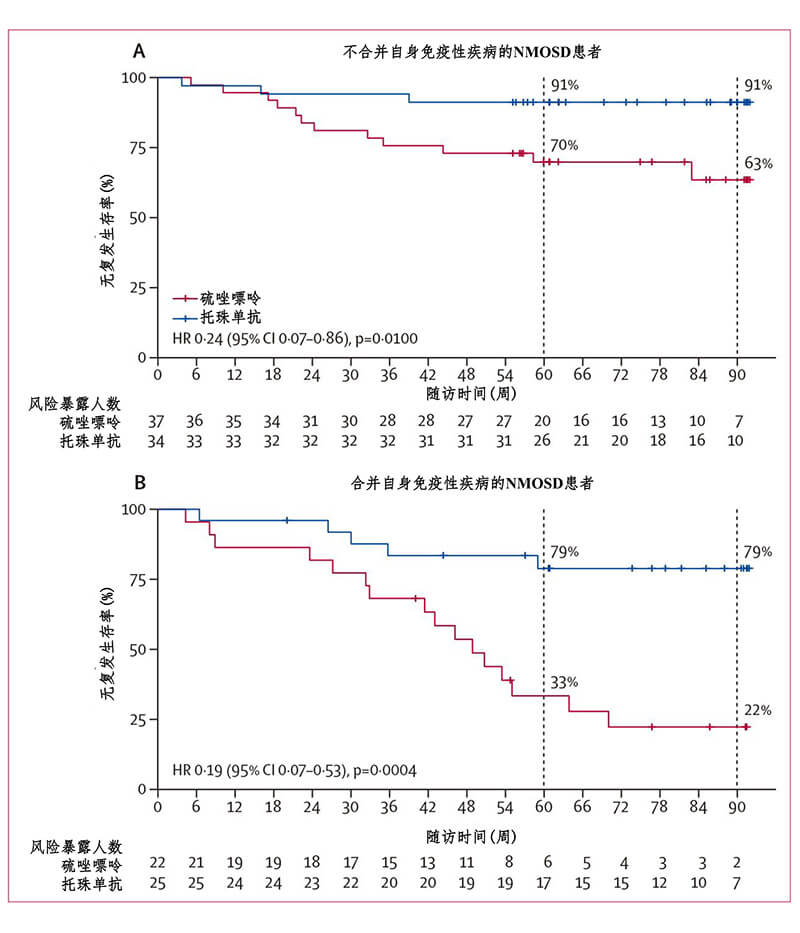
Figure 3. Stratified analysis of recurrence in NMOSD patients with other autoimmune diseases (full analysis set)
The main results of the TANGO trial suggest that tozumab targeting IL-6R significantly reduces the risk of NMOSD recurrence compared with azathioprine, and that tozumab may be the first choice for NMOSD patients with high recurrence rate or refractory NMOSD, especially those with autoimmune diseases. It provides a new safe and effective treatment regimen for NMOSD patients. The TANGO trial will greatly facilitate the inclusion of targeted IL-6R therapies into the international guidelines for NMOSD, which will guide neurologists worldwide on how to select new targeted therapies and traditional immunosuppressants.
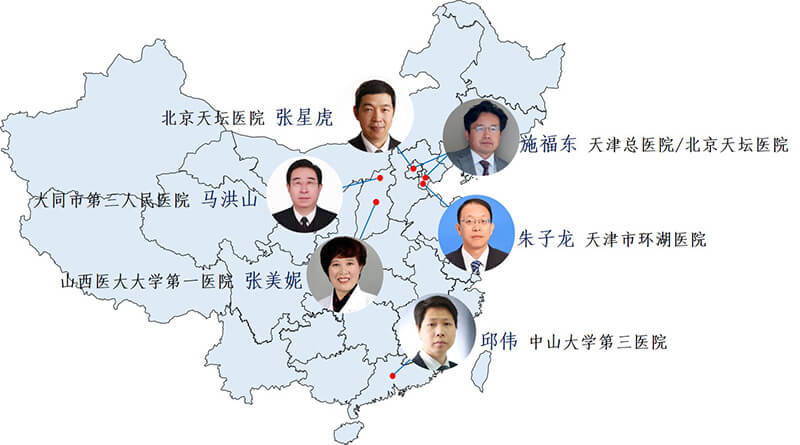
Figure 4. Distribution of TANGO experiment center and regional PI
The TANGO trial is a investigator-designed, self-funded, randomized controlled study that is independent of any pharmaceutical company and is the world's first comparative study. It was conducted by senior experts from six hospitals across the country (Figure 4). During the two-year clinical trials, lead author juck doctors in six hospitals between several reciprocating supervision, coordination, ensure the high quality of clinical trials and the consistency of multicenter trials, and his research team at the international conference on behalf of the TANGO experiment published research results (figure 5), is widely recognized in international peers and support.
图5. 论文第一作者张超医生在第35届欧洲多发性硬化治疗与研究大会(ECTRIMS)上代表TANGO试验研究者做大会主题报告(瑞典,斯德哥尔摩,2019.9.12)
The design and implementation of TANGO experiment are strictly in accordance with international standards. Unlike the three clinical trials, TANGO patients were included according to the latest NMOSD diagnostic criteria based on international expert consensus in 2015. In addition to classic endpoint events such as clinical recurrence and disability progression, the TANGO trial also used objective visual assessment indicators such as visual evoked potential (VEP) and optical coherence tomography (OCT) and nuclear magnetic resonance imaging (MRI) to assess lesion and brain and spinal volume. More importantly, TANGO assay quantitatively detected Aquaporin 4 (AQP4) autoantibody in serum of patients by transfected cell immunofluorescence (CBA) method. The method by a biological technology co., LTD. Tianjin tianhai new field and the nerve center of immune of strict quality control, can learn about medication during the dynamic changes of pathogenic autoantibodies, provides a direct basis, to evaluate the effect of drugs at the same time for the next NMOSD diagnosis, condition monitoring and clinical trials in patients with established mature, methodology foundation of international recognition.
TANGO experiment is an in-depth exploration of B-cell targeted therapy for multiple sclerosis, neuromyelitis spectrum disease, myasthenia gravis and other diseases by Professor Shi Fudong's team in the past 10 years. Since 2010, the team has established a low-dose sequential therapy of rituximab, an anti-CD20 monotherapy, to monitor the effect of b-cell removal by flow cytometry, which has effectively controlled the recurrence of neuromyelitis spectrum disease and significantly reduced the annual treatment cost of patients. The regimen is being used in a number of neurological centers in China, South Korea and Japan, benefiting an increasing number of patients. For some patients with poor therapeutic effect of rituximab, Dr. Zhang chao, as the main researcher, introduced bortezomib, a proteasome inhibitor, as an additional therapy to control the disease recurrence of refractory patients by deleting plasma cells producing pathological antibodies (JAMA Neurology, 2017). Tozumab, on the other hand, does not delete B cells or plasma cells, but achieves a state of immune balance by inhibiting the activity of self-reactive B cells (Figure 6). At present, the team is making in-depth studies on B cells in peripheral blood and CEREBROspinal fluid by using single-cell sequencing and multi-omics techniques, in an attempt to comprehensively elucidate the mechanism of B cells' role in NMOSD, and to lay a theoretical foundation for more precise b-cell targeted therapy.
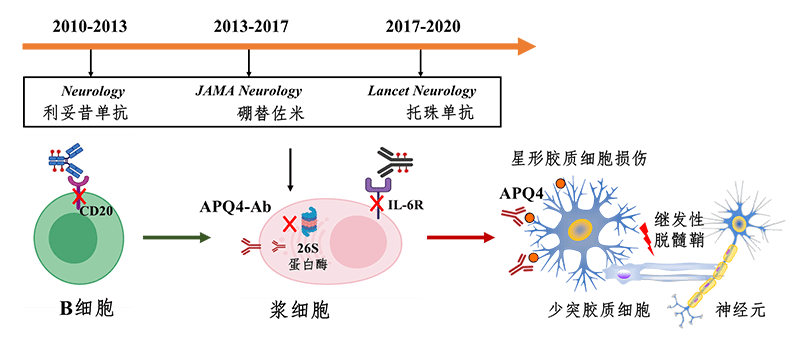
Figure 6. Exploration of targeted cell NMOSD therapy by Beijing-Tianjin Neuro-Immune Team -- a 10-year journey
TANGO trial provides a new concept of scientific and systematic prevention of disease recurrence for NMOSD patients, which is the crystallization of China's cooperation in the field of neuroimmunization and shows China's clinical research level in the field of global neuroimmunization. In addition, The revision of The Lancet Neurology was made during The severe epidemic of Novel Coronavirus disease (COVID-19) in China. In The end, The article highly praised The selfless dedication and self-sacrifice of medical staff from Tianjin General Hospital, Beijing Temple of Heaven Hospital and other hospitals in China in their fight against The novel Coronavirus disease.
Figure 7. Professor Shi Fudong (center) looks at a patient with febrile optic neuromyelitis on January 23, 2020
Introduction to the principal investigator of the TANGO experiment
Professor Shi Fudong, chief physician, General Hospital of Tianjin Medical University, Beijing Temple of Heaven Hospital, Beijing-Tianjin Neuroimmunization Center (Figure 7)
Doctor of Neurology, Karolinska Institute, Sweden, specializes in the diagnosis, treatment and research of neuroimmune diseases, including multiple sclerosis, optic neuromyelitis, myasthenia gravis and other diseases. Shi has completed clinical and scientific training at Harbin Medical University, Peking Union Medical College Hospital, Karolinska Institute, Scripps Research Institute and Barrow Neurological Institute in the United States. He is a distinguished Professor of "Changjiang Scholar", chief scientist of The National Major Scientific Research Program (973), chairman of the Pan-Asian Multiple sclerosis Therapy and Research (PACTRIMS) Scientific Committee, group leader of neuroimmunology group of the Neurology Branch of the Chinese Medical Association, and editorial board member of Science Medicine. According to independent analysis by Elsevier, the world's largest publisher, Over the past five years Shifu Dong has ranked 20th globally in scientific and technological output in areas related to neuroinflammation, with the 5th highest citation rate and H index of 41.
Reference