The other diagnostic products based on autoantibody detection depend on the CBA-TAS technology platform. It is facilities the diagnosis majority of autoimmune diseases such as CNS demyelination, myasthenia gravis, autoimmune encephalitis, paraneoplastic nerve syndrome, the node of Ranvier, neuronal axon injury, myositis, and autoimmune hepatitis.
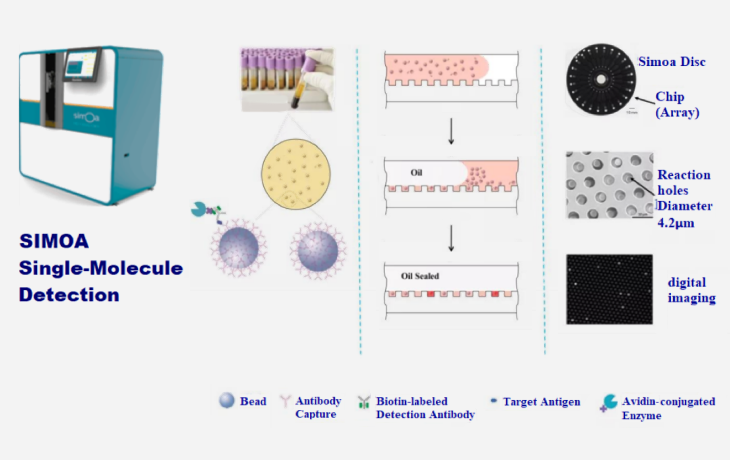
The specific biomarkers that include p-Tau181, p-Tau 231, α-synuclein, Nfl and Aβ, and other testing products for the detection of the neurodegenerative disease (Alzheimer’s Disease & Parkinson’s Disease) are relying on the Simoa which is a highly sensitive single-molecule technology platform.
The detection of the cytokine storm factors (MMP9, MCP1, NFH, NFL, IL-1, IL-10, IL-6, TNF-α) are designed by the Ella technology platform aimed to the brain injury identification.

Myasthenia gravis (MG) is a kind of acquired transmission disorder at neuromuscular junction mediated by autoantibodies. Clinically, MG patients show fluctuating symptoms of skeletal muscle fatigue and weakness, and autoantibody detection is one of the important auxiliary tests for the diagnosis of MG. Some countries and regions take AchR and MuSK antibodies as the preferred indicators for the auxiliary diagnosis of MG suspected patients. In recent ten years, studies have shown that the detection of AchR and MuSK antibodies by cell-based assay has higher sensitivity and specificity than Radioimmunoprecipitation assay (RIPA) and enzyme-linked immunosorbent assay (ELISA)1-8. This kit adopts cell-based assay with tyramine signal amplification. At present, through the methodological comparison study of 1500 myasthenia gravis cohort, it is found that the sensitivity of CBA-TSA method is improved by 10% compared to RIPA assay. The detection contents of this kit include pathogenic antibodies of myasthenia gravis (AchR, musk and Lrp4) and common thymoma antibodies (titin and RyR) for qualitative diagnosis, disease classification, diagnosis and treatment tracking of myasthenia gravis.

Central nervous system demyelination autoantibody detection kit is used for auxiliary diagnosis and differential diagnosis of demyelinating disease of central nervous system. This kit mprehensively detects AQP4, MOG, GFAP and MBP autoantibodies, which is helpful for the auxiliary diagnosis of demyelination disease. AQP4, MOG, GFAP or MBP protein antigens were expressed in carrier cells by the classical cell transfection method, which maintained the spatial conformation of antigens better. The corresponding autoantibodies in serum or cerebrospinal fluid were specifically detected by the combination of antigen and antibody, and two-color fluorescence, increased internal reference control, to ensure more accurate results, so as to achieve high sensitivity and specificity. This product adopts the improved 96-well plate process, which can be disassembled according to the test items to meet different clinical testing needs.

The autoimmune encephalitis rapid detection kit is used to aid the diagnosis of NMDAR encephalitis, marginal encephalitis and encephalitis diseases of unknown cause. This product detects autoimmune encephalitis(NMDAR、LGI1、GABABR、AMPA1、AMPA2、CASPR2、IgLON5、DPPX、DRD2、mGluR1、mGluR5、GAD65、GlyR1、Neurexin-3α)autoantibodies simultaneously, which is helpful for the auxiliary diagnosis of autoimmune encephalitis disease. Target protein antigens were expressed in carrier cells by classical cell transfection method, which maintained the spatial conformation of antigens better. The corresponding autoantibodies in serum or cerebrospinal fluid were specifically detected by the combination of antigen and antibody, two-color fluorescence, increased internal reference control, to ensure more accurate results, so as to achieve high sensitivity and specificity. This product adopts the improved 96-well plate process, which can be disassembled according to the test items to meet different clinical testing needs.
Aging causes the increase of the prevalence rate of many critical diseases and the degeneration of human body functions such as heart or brain disease and tumor. Besides the body aging caused by life time growth, the interference of psychological reasons and environmental factors can also accelerate senescence. In the process of human body cell senescence, cell proliferation stagnation, apoptosis resistance and senescence related secretion phenotypes may occur, which causes energy metabolism disorder, protein homeostasis disruption and other phenomena. Using highly sensitive humoral body fluid markers detecting techniques such as Simoa, ELLA and Luminex, we can accurately monitor various senescence related molecules (IL-1β, TNF-α, IL-6, NFL, GFAP, uPA-1, etc.) in human blood and cerebrospinal fluid samples, so as to accurately quantify the sub-health status of adults, predict the risk of critical diseases associated with aging, assist in personalized treatment, and help to build personal aging health profiles.